Seeing Into the Quantum Heart of Matter
When I moved to St Andrews as a long-haired, clean-shaven 17-year-old, it wasn’t to embark on a career as a quantum theorist – my degree was originally to be in astrophysics. For two years, I was an astrophysics student, before eventually ditching space in favour of quantum physics.
There were two main reasons for this. The first is that quantum mechanics is mindblowing, and as soon as I saw it I knew I wanted to study it more. The second is that I grew disillusioned with the lack of control in astronomy. You’re at Nature’s mercy, and you can’t re-run an experiment to get new data. Sure, you can continue to observe your star/planet/whatever, but in some cases (like the infamous ‘wow signal’) you might never see the same thing again, and you might never know for sure what happened.
I grew attracted to condensed matter physics because it has a fast turnaround between experiment and theory. The incredible control that experimenters have over their equipment means you can really probe a theory in detail to test its predictions, and often find wholly new physics while doing so. In my experience so far, nothing epitomises this control better than the quantum gas microscope. (Normally I’d link to the Wikipedia page at this point, but there isn’t one…)
An illustration of a quantum gas microscope setup – the atoms (red dots) are in a 2D plane with the lens above them.
A quantum gas microscope is a device that lets us look at individual atoms in a gas of ultracold atoms, and see how they behave in a quantum environment. Let’s back up a bit and take this step-by-step.
Ultracold Atoms
‘Ultracold atoms’ is, for once, a bit of physics terminology that does exactly what it says on the tin. It’s a gas of atoms (usually rubidium or lithium) that are cooled down to a few millionths of a degree above “absolute zero using a combination of magnetic trapping and laser cooling.
How we cool the atoms is a matter for another day – I spent one summer working in a lab doing exactly that, and it’s not an easy process. In fact, it’s a process that won a Nobel Prize in 1997 for its inventors. (There’s a movie of the process here, if you’re interested.)
Instead, let me tell you what we do with them once they’re cold. Temperature is really just a measure of the movement of particles, and if we want complete control over them, we need to cool them down so they stop jiggling around and stay as still as possible. By removing these thermal fluctuations, all that’s left is their bare quantum mechanical behaviour, which is what we usually want to study. Once cold, we can use lasers to manipulate the atoms. Depending on the atomic species, they can be drawn either to light or to dark patches in a laser beam. By shaping our laser beam into, say, a grid of bright dots (or any other pattern you like), we create an setup called an optical lattice, reminiscent of the “crystal lattice that electrons in real materials find themselves in.

Example patterns of light that can be made using a Spatial Light Modulator (SLM) device, developed by my colleagues at the University of St Andrews. Atoms are drawn to the bright spots, so by making these patterns with light, we can place the atoms in any arrangement we wish.
Now, we hit a subtlety – in a real material, it’s the electrons that move within the scaffolding of a crystal lattice, with the lattice sites marked out by the locations of atomic nuclei. In an optical lattice, it’s the laser beam that provides the scaffolding, and the atoms themselves that move around on it. Despite this, however, we find that the atoms in an optical lattice obey largely the “same “physics as electrons in a crystal lattice. This means we can use ultracold atoms to simulate the behaviour of electrons in solids. And by itself, that’s neat, but there’s more to it than that.
The Quantum Gas Microscope
Atoms are bigger than electrons, which means they’re a little easier to manipulate. In particular, this allows us to actually image them using a technique known as fluorescence imaging. Essentially, we fire a new laser beam at the atoms and measure where the photons bounce back from, allowing us to precisely locate the atoms. (In reality, it’s a quantum process where they absorb and re-emit the photons, so don’t take the phrase ‘bounce back’ literally, but I think it’s a helpful analogy.)
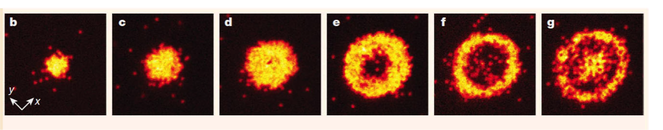
Some real images from a quantum gas microscope – the bright dots are individual atoms. The uniformly-filled regions show the atoms in a Mott insulating phase. (Image from Sherson et al. (2010), reproduced here under Fair Use Policy.)
Being able to really see the atoms in a lattice is an incredible step forward in our ability to peer into the quantum heart of matter. Take, for example, a phase of matter known as a Mott insulator, a material where there is precisely the same number of atoms fixed in place on every lattice site: with a quantum gas microscope, we can take a picture of a material and see with our own eyes that this is exactly what happens – no fancy analysis techniques required! Or, take the superfluid phase where there’s essentially a completely random number of atoms on every lattice site at any given time, since they’re all flowing around the system like a liquid – we can image that too, and every time we re-run the experiment we see the atoms are in a different place, signifying that they are moving freely around the lattice just as we’d expect.
We can even go further and add random impurities into the system, triggering a phase of matter known as the Bose glass where we’d expect to see a mixture of Mott-insulating (i.e. regular, ordered looking regions) and superfluid regions (with a random-looking distribution of particles). Mathematically, this is hard to describe, but with a quantum gas microscope, it’s easy – you can literally take a picture and see how the atoms divide into ordered and disordered regions.
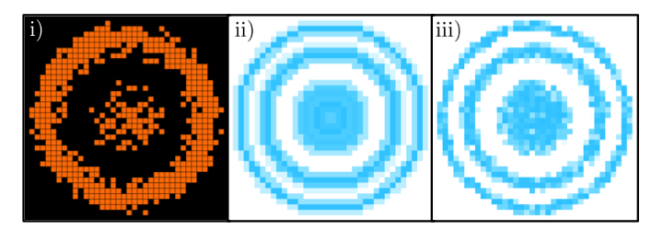
Some theoretical work done by my colleagues and I, showing what a quantum gas microscope would see in a disordered optical lattice (left) and calculations of which regions of the simulated image contain a phase of matter known as the Bose glass, shown in blue. (Image from my PhD thesis using data from Thomson et al. (2016).)
In the last few years experimental groups around the world have gone from the initial microscopes using bosonic atoms to newer ones which use fermionic atoms – this is important because electrons in real material are fermions, and if we want to simulate all of their properties, we need to do so with fermionic atoms. This means we can now get closer to replicating beguiling materials such as the infamous high-temperature superconductors, the cuprates. In a real solid, you’re stuck with what Nature gives you, but in an ultracold atoms setup, you have complete control over everything: you can tune the parameters of your material in any way you desire to investigate its behaviour in a way that you just can’t do in solid state physics.
Some researchers are a little dubious of quantum gas microscopes, taking the point of view that they’re only good for mimicking other more interesting materials and that so far, all they’ve done is confirm things we already know. A few years ago, that might have been true, but I think that now we’re entering the age where quantum gas microscopes are about to open up a whole new world of new physics where we have complete control over our quantum systems and can start to engineer and test all sorts of exotic pieces of physics.
All in all, it’s an exciting time to be working on ultracold atoms!
Image sources: 1, 2, 3, 4. Images that I do not own the copyright to are reproduced here under what I believe to be the terms of the Fair Use Policy for educational use and are credited and linked to accordingly – should the owners of the copyright disagree, feel free to drop me an e-mail and I’ll remove them. The cover image for this article was sourced from here, and I believe it to be part of a press release that may be reproduced freely.
